
Intraoperative solution to reduce scar formation after surgery
TetraDerm
TetraDerm has been developed using the Tetramatrix™ platform technology and can be used in any surgical incision site to reduce scarring. TetraDerm is a flowable matrix used during surgery to provide internal cushioning, reduce mechanical tension and dead space and thus minimising scar formation.
Internal cushion to decrease tension and deadspace to reduce scar formation
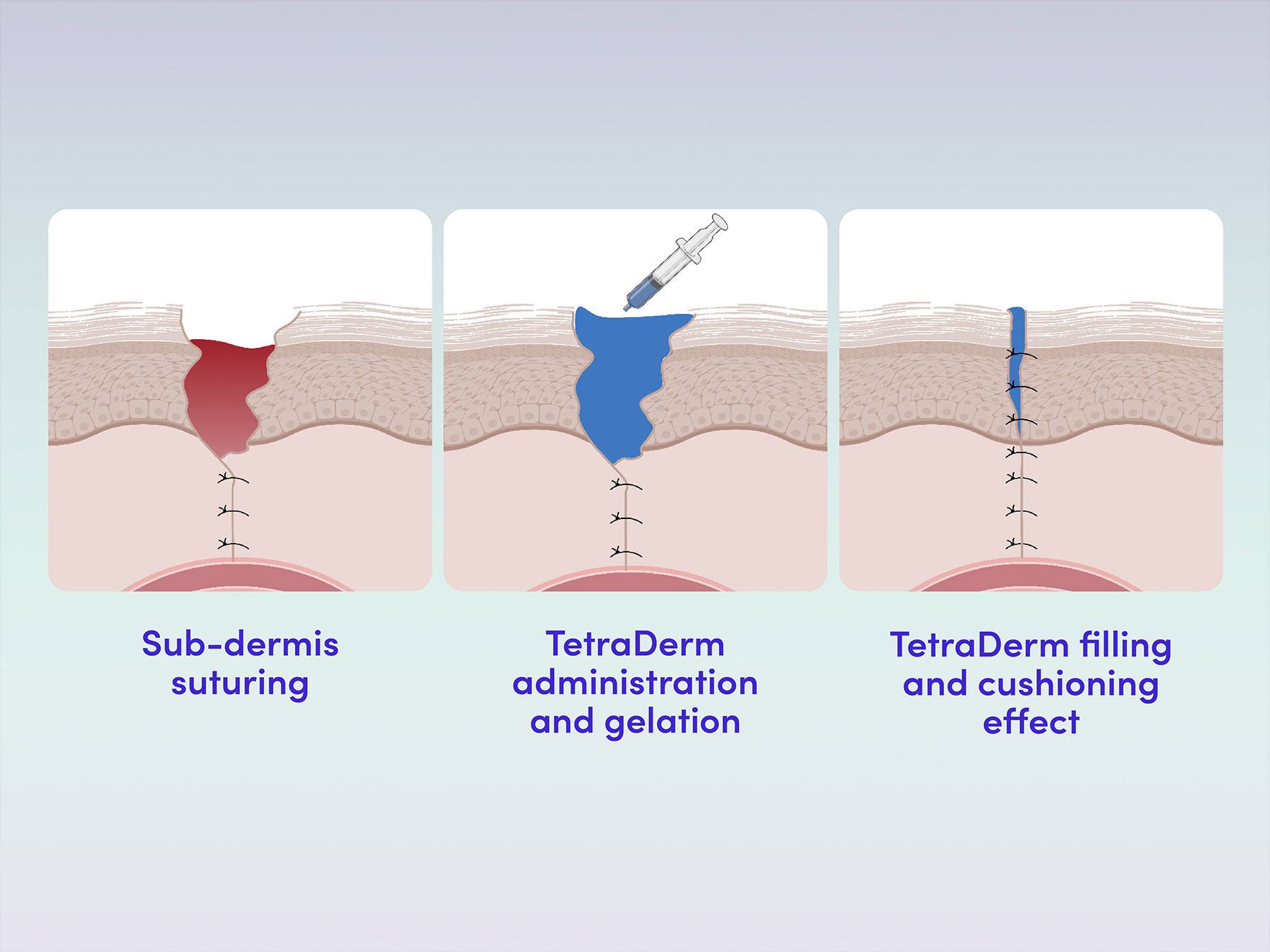
Flowable matrix solution for deep and superficial sites
The derivative product from the Tetramatrix™ platform technology is provided to clinicians in a ready-to-use format. There is no need for preparation and it is easy-to-use without impacting clinical workflow. The product is a flowable dermal matrix that forms a uniform matrix structure within dermal layers without the need for any external stimuli (such as UV light or chemical reactions) gelation is triggered by physiological temperature. The resulting matrix and its transition process allow full coverage of the site, regardless of its shape, geometry or topography. After matrix formation the product physically and biologically integrates within the dermal tissue.
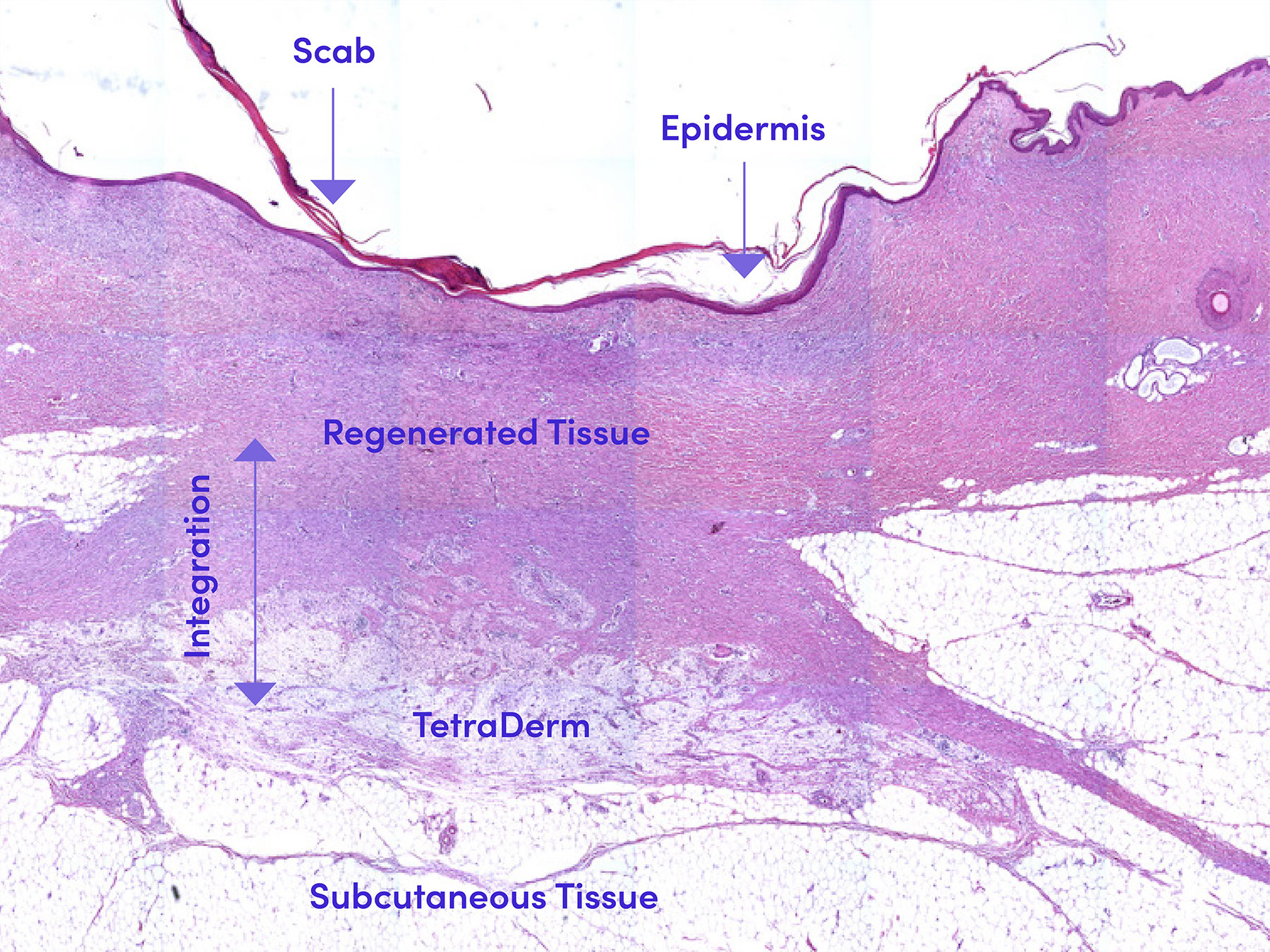
Physically and biologically integrates within the site
In a full-thickness, dermal defect preclinical model, 5x5cm wounds were filled with the product. The solutions successfully transitioned into a matrix and fully filled the defect sites. The product molds within the boundaries of the defect and under mechanical tension with movement, the product remains within the defect based on the elastic and adhesive nature of the products derived from the Tetramatrix™ platform technology. Histologically the product integrates within the host environment due to the biomimetic properties of the Tetramatrix™ platform technology.
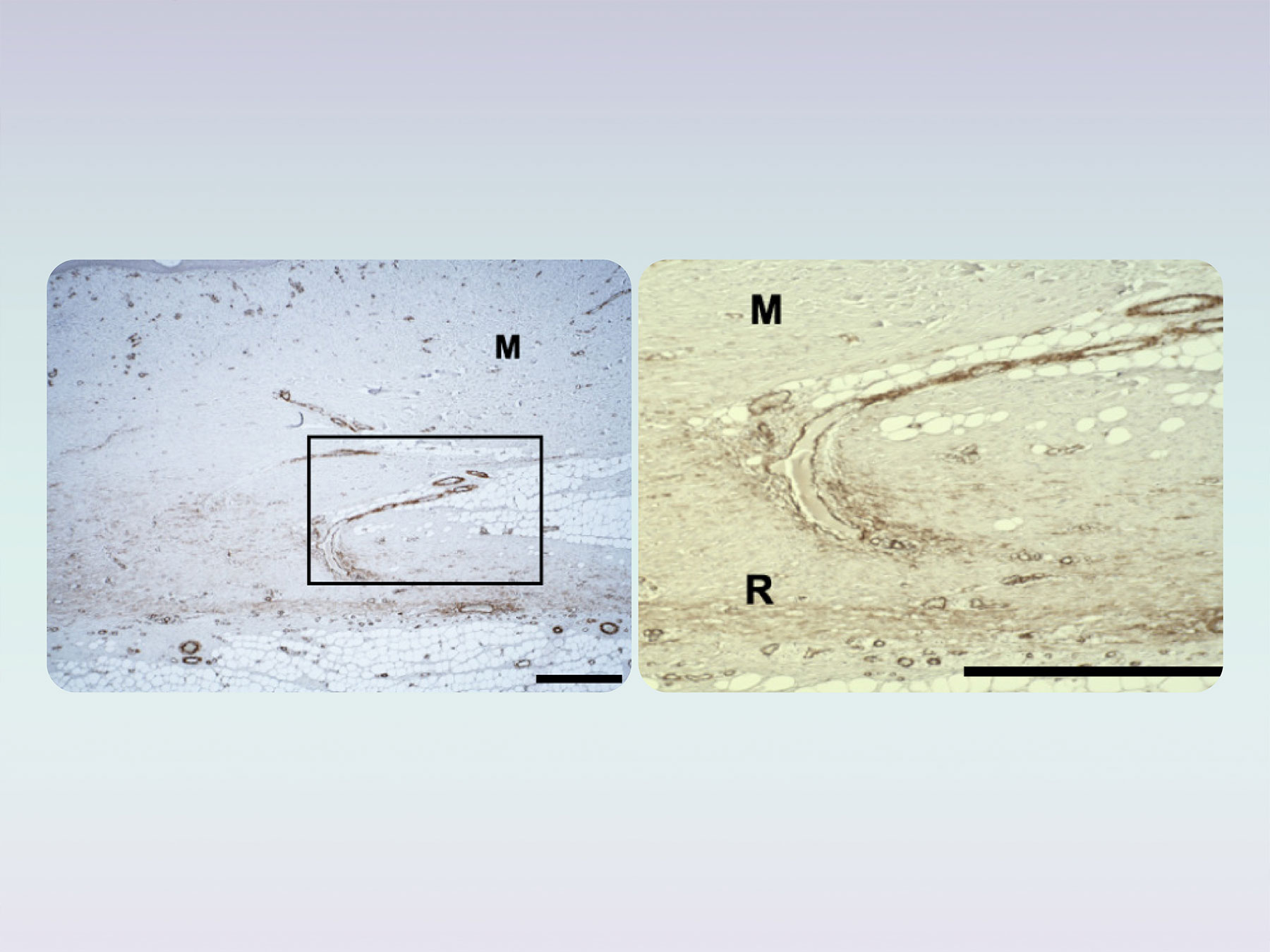
Supports natural soft tissue healing
Histological evaluations in preclinical studies of the product derived from the Tetramatrix™ platform technology showed that in dermal sites the product supports natural tissue healing. The biomimetic properties of the product allow integration of the host mature (M) tissues within the regenerated (R) sites, which may lead to the reduction of scar formation in surgical sites. Currently, the product is under clinical investigation for scar reduction in surgical sites.
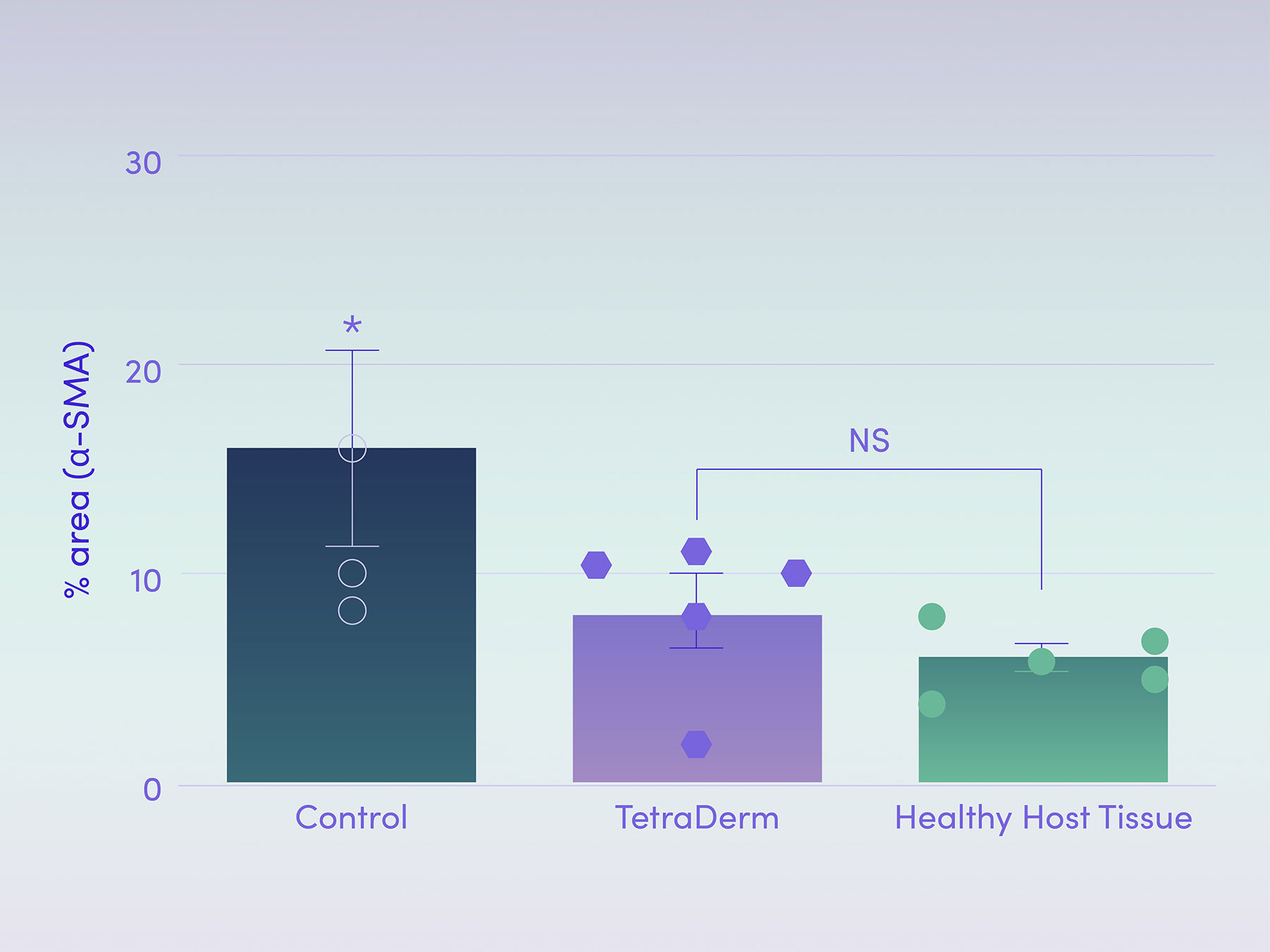
Reducing protein expression demonstrates maturation of skin remodelling
The amount of a-SMA (alpha-smooth muscle actin) at multiple sites was measured in a full-thickness, dermal defect model to measure the maturity of the skin remodelling and the activity of a specific cell type that is responsible for scar formation. The results showed that the use of the product derived from the Tetramatrix™ platform technology decreases a-SMA to the level that was seen in healthy host tissue which demonstrates the the product decreasing myofibroblast activity that may indicate reduction in scarring.